The FDA’s Mandate is Not to Maximize Lives Saved
Reducing disease and increasing longevity is not the congressional mandate of the FDA. It is to ensure the “safety and efficacy of drugs and medical devices.” The FDA could fulfill its order perfectly by rejecting all new drugs, which would 100% guarantee that an unsafe or ineffective drug never gets on the market again.
A mandate preferable to the 60 million people that die every year and the 2.5 billion people suffering from chronic diseases would be to maximize the average healthy human lifespan.
The Health Explosion
For thousands of years, everything sucked, and the average human life expectancy was 28. Then something happened around 1800, and life expectancy exploded.
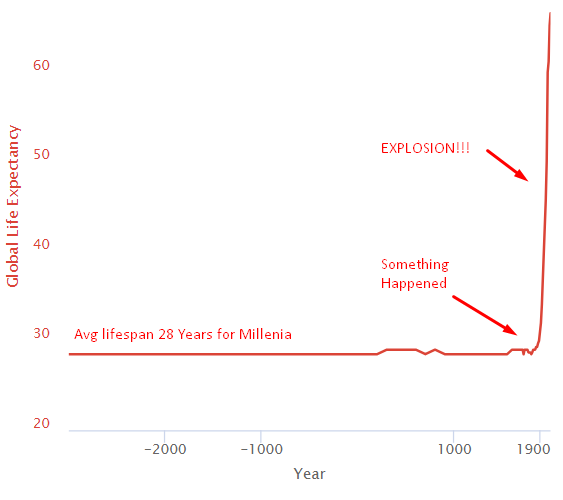
Structural Changes Leading to the Health Explosion
Let’s zoom in and see what happened in the last centuries leading to this explosion.
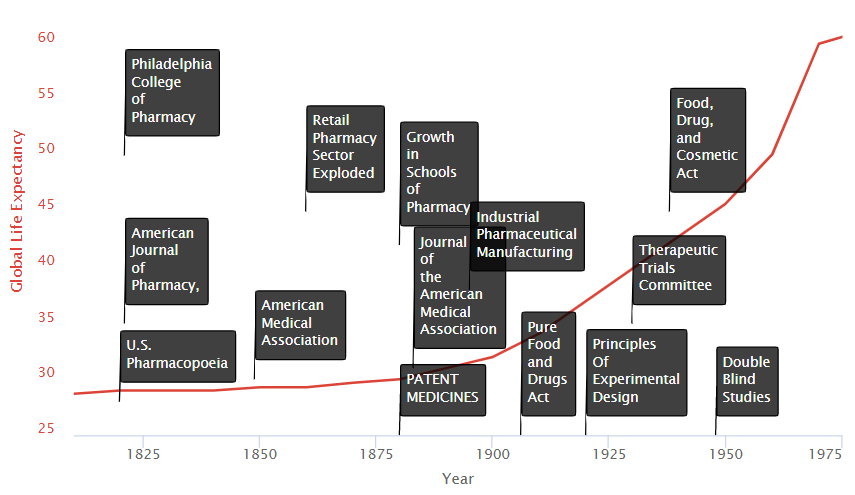
We see on the timeline two primary groups of factors:
- Research Improvements
- Incentive Improvements
Research Improvements
Schools of Medicine
An influx of new state-affiliated pharmacy schools in the 1880s and 1890s. The growth in schools of medicine trained physicians and researchers in the systematic collection of clinical data on the safety and efficacy of treatments. They also served as hubs where scientists could share new medical discoveries.
Medical Journals
New medical journals, such as the Journal of the American Medical Association (JAMA), began to disseminate discoveries widely.
AMA Drug Certifications
In 1905, the AMA formed its own Council on Pharmacy and Chemistry, which levied a fee on manufacturers to evaluate their drugs for quality (ingredient testing) and safety. Drugs accepted by the Council could carry the AMA’s Seal of Acceptance. The AMA’s Chemical Laboratory tested commercial statements about the composition and purity of drugs in their labs. The Council on Pharmacy and Chemistry followed up with safety
and rudimentary efficacy evaluations designed to eliminate exaggerated or misleading therapeutic claims. Drugs that provided symptomatic relief were awarded the AMA’s Seal of Acceptance.
Cooperative Investigations
During the 1920s, ’30s, and ’40s, medical researchers began to conduct “cooperative investigations.” These were designed to overcome errors attributed to individual observers working in relative isolation and instead replaced them with standardized evaluations of therapeutic research in hundreds of patients.
Freedom to Try
Therapeutic experimentation did not begin to gain a proper foothold in modern medicine until the U.S. legal system stopped equating experimentation with medical malpractice. In 1935, the state of Michigan authorized controlled clinical investigations as a part of medical practice without subjecting the researcher to strict liability (without fault) for any injury, so long as the patient consented to the experiment. In particular, the Court accepted that experimentation was necessary not just to treat the individual but also to help medicine progress. “We recognize,” noted the Court, “that if the general practice of medicine and surgery is to progress, there must be a certain amount of experimentation carried on.”
Incentive Improvements
Medical Patent Protections & Mass Production
In the late nineteenth and early twentieth century, interest in clinical objectivity grew, spurred on by astounding successes in laboratory science and clinical medicine abroad (e.g., the discovery of microbes, pasteurization of milk, development of anthrax and rabies vaccines). International recognition of medical patents contributed to a boom in large-scale industrial pharmaceutical manufacturing led by Bayer and Pfizer. In 1880, patent medicines constituted 28% of marketed drugs; by 1900, however, they represented 72% of drug sales.
The industrial revolution gave way to technologies allowing for mass production and distribution on a global scale. This method was vastly more efficient and profitable than traditional means, and these greater profits drove more significant investment and, thereby, greater medical progress.
The History of Drug Regulation
In the 1800s, snake oil salesmen would make false claims about the concoctions they were selling. The original solution was the AMA Council on Pharmacy and Chemistry certification to ethical drug products that met their standards.
1906 – Ingredient Lists and No Lying on Labels
The 1906 Pure Food and Drugs Act empowered the Bureau of Chemistry (forerunner of the FDA) to seize adulterated and misbranded products. The law also prohibited “false and misleading” statements on product labels. The rule listed eleven so-called “dangerous ingredients,” including opium (and its derivatives) and alcohol which, if present in the product, had to be listed on the drug label. This listing requirement alone inspired many manufacturers to abandon the use of many “dangerous ingredients” following the passage of the 1906 Act.
1938 – Safety Trial Requirements
The Federal Food, Drug, and Cosmetic (FDC) Act of 1938 is passed by Congress, containing new provisions:
- Extending control to cosmetics and therapeutic devices.
- Requiring new drugs to be shown safe before marketing
Manufacturers were required to demonstrate to FDA that they had carried out all reasonably applicable studies to demonstrate safety and that the drug was “safe for use.”
1950’s – FDA Protects Americans from the Thalidomide Disaster
In the late 1950s, thalidomide became available in 46 countries (but not the U.S.) to treat nausea associated with morning sickness during pregnancy. Unfortunately, the potential side effects were not fully understood. As a result, thousands of children were born with congenital disabilities, most notably phocomelia (limb malformations).

Fortunately, the existing 1938 FDA safety requirements completely protected Americans from the fate of Europe. However, amid the terrifying news reports of the tragedy, the U.S. government felt compelled to do something in response.
1962 – Kefauver Harris Efficacy Amendment.
As effective safety regulations were already in place, the government chose to restrict the production of new treatments, primarily by requiring extensive additional efficacy testing via the 1962 Kefauver Harris Amendment.
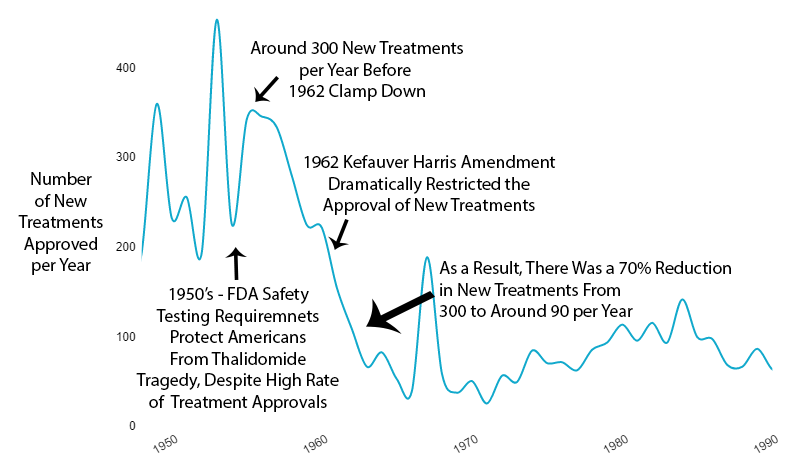
Results of Increased Restrictions
Reduction in New Treatments
The new regulatory clampdown on approvals immediately reduced the production of new treatments by 70%.
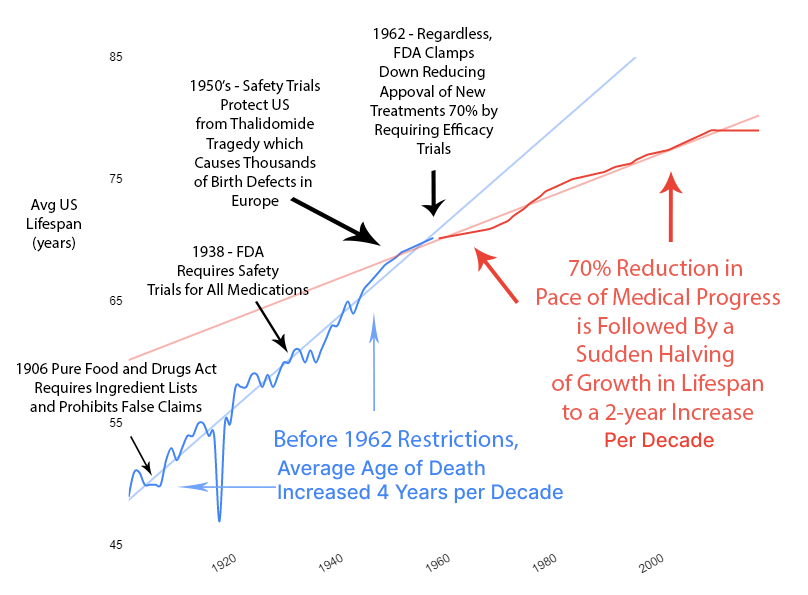
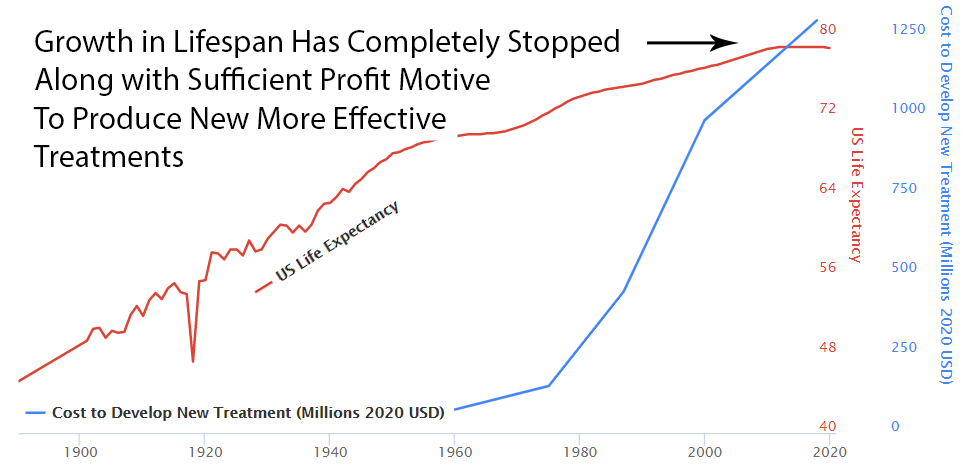
Slowed Growth in Lifespan
Over the previous 50 years, rapid advancements in medical science had produced a 4-year increase in human lifespan every decade. This amazingly linear growth rate had followed millennia with a flat human lifespan of around 28 years. Following this new 70% reduction in the pace of medical progress, the growth in human lifespan was immediately cut in half to an increase of 2 years per decade.
Diminishing Returns?
You might say, “It seems more likely — or as likely — to me that drug development provides diminishing returns to life expectancy.” However, diminishing returns produce a slope of exponential decay. It may be partially responsible, but it’s not going to create a sudden change in the linear slope of a curve as linear as life expectancy was before and after the FDA.
Additionally, we’re only three lifetimes from George Washington. The modern scientific method has only been applied to medicine for .0001% of human history. However, the more clinical research studies one reads, the more one realizes how little we know. We know almost nothing compared to what will eventually be known about the human body. The currently highly restrictive method of clinical research makes rapid discovery impossible. We’re at the very beginning of thousands or millions of years of systematic discovery. So it’s unlikely that this decline in lifespan growth resulted from diminishing returns due to our running out of things to discover.
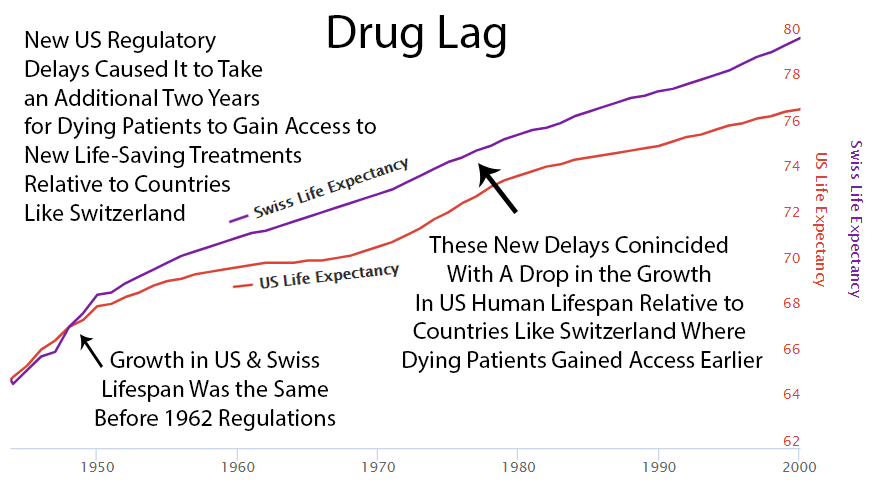
2 Year Drug Lag
It currently takes over ten years for a life-saving drug to make its way through the FDA’s regulatory process and become available to dying patients. Following the 1962 increase in U.S. regulations, one can see a divergence from the growth in life expectancy in Switzerland, which did not introduce the same delays to availability.
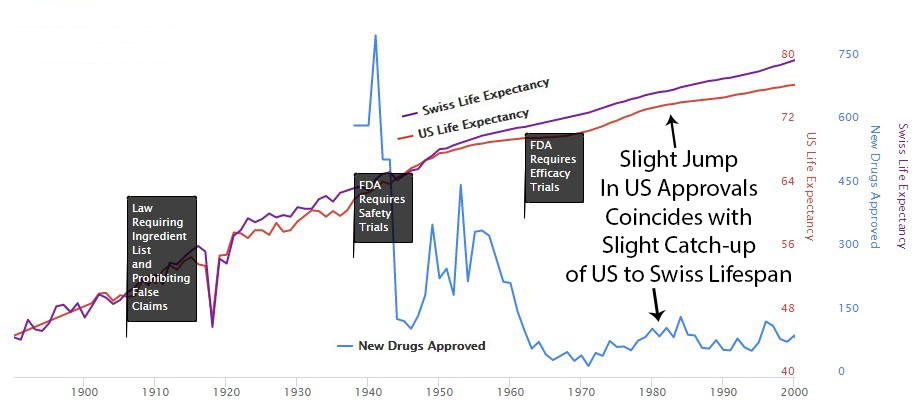
Perhaps it’s a coincidence, but you can see an increase in drug approvals in the ’80s, and at the same time, the gap between Switzerland and the U.S. gets smaller then. Then U.S. approvals decreased in the ’90s, and the gap expanded again.
Here’s a news story from the Non-Existent Times by No One Ever without a picture of all the people that die from lack of access to life-saving treatments that might have been.
Explosion in Costs
Since 1962, the cost of bringing a new treatment to market has gone from $74 million to over $1 billion U.S. dollars (2020 inflation-adjusted).
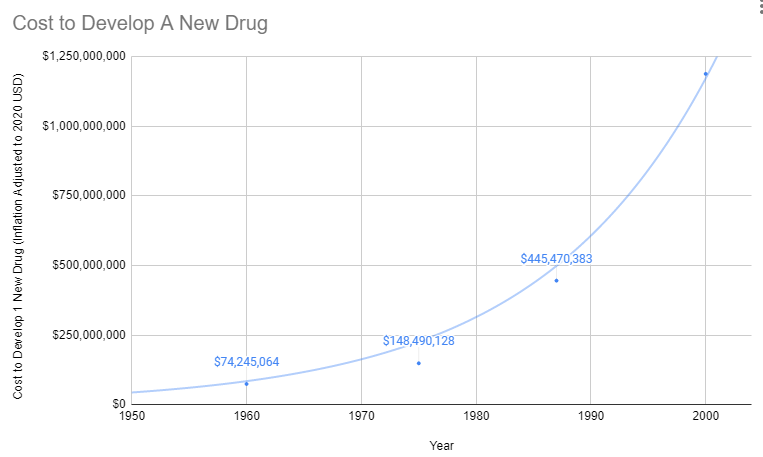
Treatments for Rare Diseases Make No Financial Sense
The costs of FDA regulations do not vary with the number of potential patients, so the decline in drug development has been particularly harmful to those suffering from rare diseases. Each rare disease afflicts only a small number of people, but thousands of rare diseases exist. In aggregate, rare diseases afflict millions of Americans: according to an AMA estimate (AMA 1995), as many as 10 percent of the population. Thus, millions of Americans have few or no therapies available to treat their diseases because of increased costs of drug development brought about by stringent FDA “safety and efficacy” requirements. In response to this problem, in 1983, the Orphan Drug Act was passed to provide tax relief and exclusive privileges to firms developing drugs for diseases affecting two hundred thousand or fewer Americans (AMA 1995).
Oligopolies Protect Existing Inferior Treatments
Before 1962, a brilliant scientist could develop a new treatment, raise $74 million for safety and efficacy testing, and bring it to market. With the current cost of getting a new therapy to patients over a billion dollars, there are only a handful of companies with enough money to risk hundreds of millions on a 90% chance of rejection by the FDA. So today, the brilliant scientist goes to one of these companies, and the company buys the patent for several million dollars. It’s likely the drug company already has an existing inferior drug on the market that they’ve already spent a billion dollars getting approved.
Then the drug company has two options:
Option 1: Risk $1 billion on clinical trials
Possibility A: Drug is one of the 90% the FDA rejects. GIVE BANKER A BILLION DOLLARS. DO NOT PASS GO.
Possibility B: Drug turns out to be one of the 10% the FDA approves. Yay!!! Now it’s time to try to recover that billion dollars. However, very few drug companies have enough money to survive this game. So, this company almost certainly already has an inferior drug on the market to treat the same condition. Hence, any profit from this drug will likely be subtracted from revenue from existing, less effective drugs they’ve already spent a billion dollars on.
Option 2: Put the patent on the shelf
Do not take a 90% chance of wasting a billion dollars on failed trials. Do not risk making your already approved cash-cow drugs obsolete.
What’s the benefit of bringing better treatment to market if you lose a billion dollars? Either way, the profit incentive is entirely in favor of just buying better treatments and shelving them.
The current flat line in US lifespan growth and chronic disease burden is completely consistent with a system that completely disincentivizes medical progress.
Efficacy Trials Before 1962
A given drug may be effective at treating hundreds of conditions. However, knowing which conditions a drug may improve is impossible until real-world patients try it.
Before the 1962 regulations, it cost a drug manufacturer an average of $74 million (2020 inflation-adjusted) to develop and test a new drug for safety before bringing it to market. Once the FDA had approved it as safe, efficacy testing was performed by the third-party American Medical Association.
The AMA would collect data from its 144,000 physicians on the efficacy of different drugs for various conditions. Once it was determined which conditions a drug was or was not effective for, the results would be published in the Journal of the American Medical Association (JAMA). The AMA would only give its Seal to drugs that it had determined safe and effective for specific conditions.
The 1962 regulations made these large real-world efficacy trials illegal. Ironically, even though the new rules were primarily focused on ensuring that drugs were effective through controlled FDA efficacy trials, they massively reduced the quantity and quality of the efficacy data that was collected for several reasons:
- New Trials Were Much Smaller
- Were Far More Expensive
- Participants Were Less Representative of Actual Patients
- They Were Run by Drug Companies with Conflicts of Interest Instead of the 3rd Party AMA
Exclusion Criteria
Subjects in FDA Trials Are Not Representative of the Actual People Receiving Treatment
External validity is the extent to which the results can be generalized to a population of interest. The population of interest is usually defined as the people the intervention is intended to help.
Phase III clinical trials are designed to exclude most of the population of interest. In other words, the subjects of the drug trials are not representative of the prescribed recipients once said drugs are approved. One investigation found that only 14.5% of patients with major depressive disorder fulfilled eligibility requirements for enrollment in an antidepressant efficacy trial.
As a result, the results of these trials are not necessarily generalizable to patients matching any of these criteria:
- Suffer from multiple mental health conditions (e.g., post-traumatic stress disorder, generalized anxiety disorder, bipolar disorder, etc.)
- Engage in drug or alcohol abuse
- Suffer from mild depression (Hamilton Rating Scale for Depression (HAM-D) score below the specified minimum)
- Use other psychotropic medications.
These facts call into question the external validity of standard efficacy trials.
The fact that co-morbidities are excluded also makes it impossible to discover potential new uses for a treatment.
New Trials Were Very Small
Due to exclusion criteria and added costs, patient sample sizes are tiny. The number of subjects per trial on average is:
- 275 patients are sought per cardiovascular trial
- 20 patients per cancer trial
- 70 patients per depression trial
- 100 per diabetes trial
Solution: Collect Quantified Self Data on Actual Patients
In the real world, no patient can be excluded. Even people with a history of drug or alcohol abuse, people on multiple medications, and people with multiple conditions must be treated. Only through the crowdsourcing of this research would physicians have access to the true effectiveness rates and risks for their real-world patients.
The results of crowdsourced studies would exhibit complete and utter external validity since the test subjects are identical to the population of interest.
Current Regulations Appear to Cause More Deaths from Delay Than They Save
Some studies have estimated that 5,000 to 10,000 lives are saved each decade by preventing dangerous drugs from making it to market. This analysis looked at pre-FDA deaths in the U.S. and deaths in foreign countries without an FDA.
In comparison, studies have shown that the added delays and cost of FDA regulatory hurdles range between 21,000 to 120,000 deaths per decade.
So the NET outcome of lives saved minus lives lost is likely between 11,000 and 115,000 additional deaths each decade due to added delays and costs of the regulatory process.
Sources
- Google Spreadsheet of FDA Spending vs. Life-Expectancy
- Summary of NDA Approvals & Receipts, 1938 to the present
- Theory, Evidence, and Examples of FDA Harm
- DATA
- GDP
- Reform, Regulation, and Pharmaceuticals — The Kefauver–Harris Amendments at 50
- Consumer Price Index
- Estimates of World GDP, One Million B.C. – Present
- Newspaper Generator
- Report suggests drug-approval rate now just 1-in-10
- How many people die and how many are born each year?
- Gross World Product per capita
- History of Clinical Trials
- How many life-years have new drugs saved?
- CATO
- Medical Innovation
- Timeline History of Clinical Research
- FDA and Clinical Drug Trials: A Short History
- Do Off-Label Drug Practices Argue Against FDA Efficacy Requirements?
- Reform Options
- Before Occupy: How AIDS Activists Seized Control of the FDA in 1988
- A Brief History of the Center for Drug Evaluation and Research
- Milestones in U.S. Food and Drug Law History

