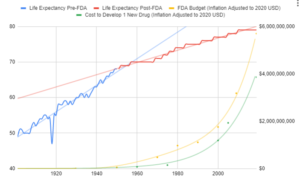
Between 2009 and 2018, the estimated median capitalized research and development cost per product was $985 million, counting expenditures on failed trials.
1893 – The Advent of Safety and Efficiency Trials
In the late nineteenth and early twentieth century, clinical objectivity grew. The independent peer-reviewed Journal of the American Medical Association (JAMA) was founded in 1893. It would gather case reports from the 144,000 physicians members of the AMA on the safety and effectiveness of drugs. The leading experts in the area of a specific medicine would review all of the data and compile them into a study listing side effects and the conditions for which a drug was or was not effective. If a medicine was found to be safe, JAMA would give its seal of approval for the conditions where it was found to be effective.
The adoption of this system of crowd-sourced, observational, objective, and peer-reviewed clinical research was followed by a sudden shift in the growth of human life expectancy. After over 10,000 years of almost no improvement, we suddenly saw a strangely linear 4-year increase in life expectancy every single year.
Since the abandonment of the former efficacy trial model, costs have exploded. Since 1962, the cost of bringing a new treatment to market has gone from $74 million to over $1 billion US dollars (2020 inflation-adjusted).
1893 – The Advent of Safety and Efficiency Trials
In the late nineteenth and early twentieth century, clinical objectivity grew. The independent peer-reviewed Journal of the American Medical Association (JAMA) was founded in 1893. It would gather case reports from the 144,000 physicians members of the AMA on the safety and effectiveness of drugs. The leading experts in the area of a specific medicine would review all of the data and compile them into a study listing side effects and the conditions for which a drug was or was not effective. If a medicine was found to be safe, JAMA would give its seal of approval for the conditions where it was found to be effective.
The adoption of this system of crowd-sourced, observational, objective, and peer-reviewed clinical research was followed by a sudden shift in the growth of human life expectancy. After over 10,000 years of almost no improvement, we suddenly saw a strangely linear 4-year increase in life expectancy every single year.
1938 – The FDA Requires Phase 1 Safety Trials
Elixir sulfanilamide was an improperly prepared sulfonamide antibiotic that caused deaths in the United States in 1937.
Congress reacted to the tragedy, by requiring all new drugs to include “adequate tests by all methods reasonably applicable to show whether or not such drug is safe for use under the conditions prescribed, recommended, or suggested in the proposed labeling thereof.”
These requirements evolved to what is now called the Phase 1 Safety Trial.
This consistent 4 year/year increase in life expectancy remained unchanged before and after the new safety regulations.
This suggests that the regulations did not have a large-scale positive or negative impact on the development of life-saving interventions.
1950’s – The FDA Requires Phase 2 Safety Trials
Thalidomide was first marketed in Europe in 1957 for morning sickness.
While it was initially thought to be safe in pregnancy, it resulted in thousands of horrific birth defects.
Fortunately, the existing FDA safety regulations prevented any birth defects in the US.
Despite the effectiveness of the existing US regulatory framework in protecting Americans, newspaper stories such as the one below created a strong public outcry for increased regulation.

1962 – The FDA Requires Phase 2 Safety Trials
As effective safety regulations were already in place, the government instead chose to respond to the Thalidomide disaster by regulating efficacy testing via the 1962 Kefauver Harris Amendment.
Prior to the 1962 regulations, it cost a drug manufacturer an average of $74 million (2020 inflation-adjusted) to develop and test a new drug for safety before bringing it to market. Once the FDA had approved it as safe, efficacy testing was performed by the third-party American Medical Association.
Following the regulation, trials were instead to be conducted in small, highly-controlled trials by the pharmaceutical
industry.
Reduction in Efficacy Data
The 1962 regulations made these large real-world efficacy trials illegal.
Ironically, even though the new regulations were primarily focused on ensuring that drugs were effective through controlled FDA efficacy trials, they massively reduced the quantity and quality of the efficacy data that was collected for several reasons:
- New Trials Were Much Smaller
- Participants Were Less Representative of Actual Patients
- They Were Run by Drug Companies with Conflicts of Interest Instead of the 3rd Party AMA
Explosion in Costs
Since the abandonment of the former efficacy trial model, costs have exploded.
The cost of development began to explode in 1962 when the FDA was granted the power to prevent new drugs from ever being sold. This also coincided with a sudden slowing in the growth of life expectancy.
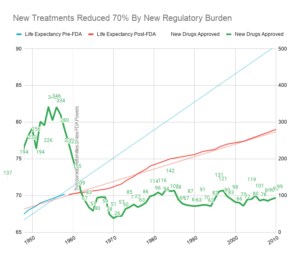
There was also a significant decline in the number of new treatments approved following the
Sources
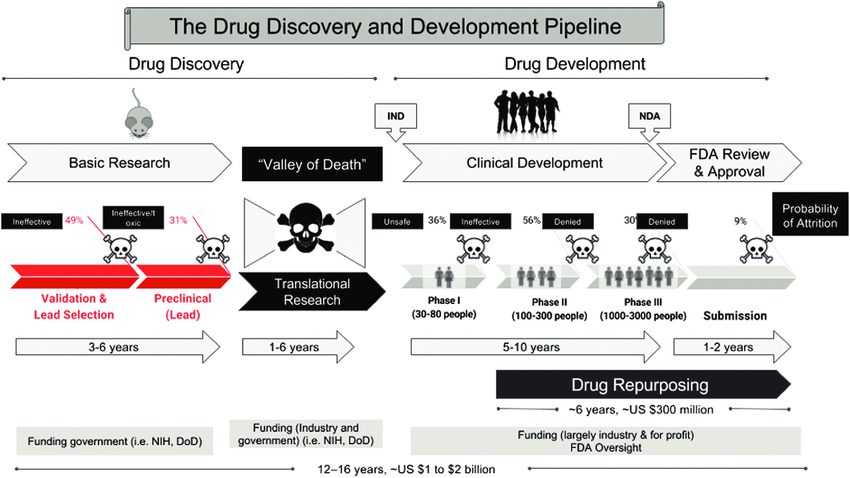
- Crossing-over-the-Valley-of-Death-The-distinct-stages-of-drug-development
- JAMA
- Google Spreadsheet of FDA Spending vs Life-Expectancy
- Summary of NDA Approvals & Receipts, 1938 to the present
- Theory, Evidence and Examples of FDA Harm
- DATA
- GDP
- Reform, Regulation, and Pharmaceuticals — The Kefauver–Harris Amendments at 50
- Consumer Price Index
- Estimates of World GDP, One Million B.C. – Present
- Newspaper Generator
- Report suggests drug-approval rate now just 1-in-10
- How many people die and how many are born each year?
- Gross World Product per capita
- History of Clinical Trials
- How many life-years have new drugs saved?
- CATO
- Medical Innovation
- Timeline History of Clinical Research


Comments